Purifico
Comparing Disinfectants
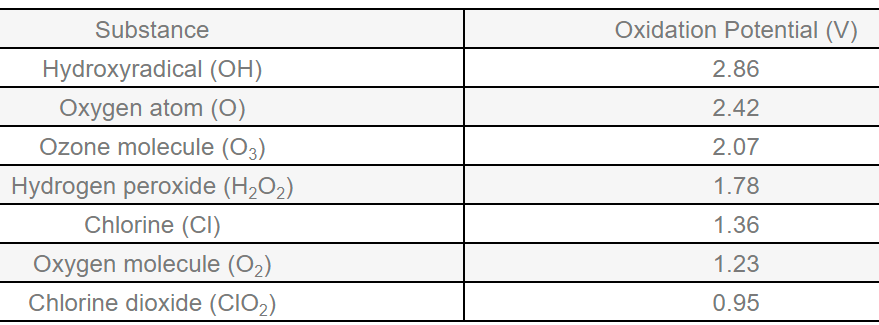
Ozone leads the way in defending against water-borne pathogens like bacteria, fungi, and viruses. Pound-for-pound, ozone outperforms other chemicals due to its strong oxidizing power. Plus, it leaves no toxic residues, allowing operators to use whatever amount is needed to get the job done. With other chemicals, toxic residues are produced which constrains dosage, allowing certain pathogens to evade destruction. When it comes to safeguarding your crops or livestock from water-borne pathogens, Purifico ozone products provide unparalleled protection and peace of mind.
Home » Comparing Disinfectants