Superior Water Treatment
Chemistry of Ozone
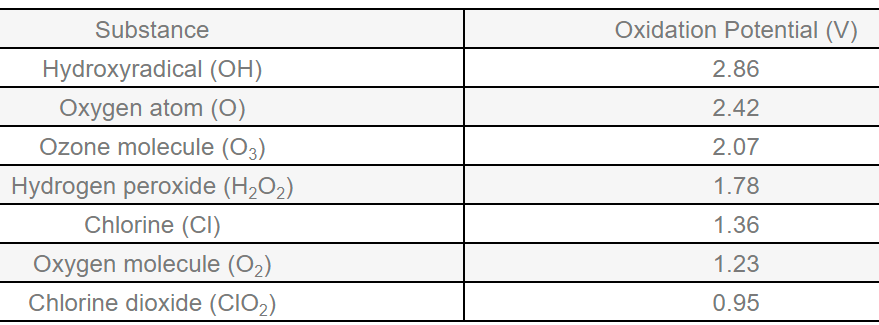
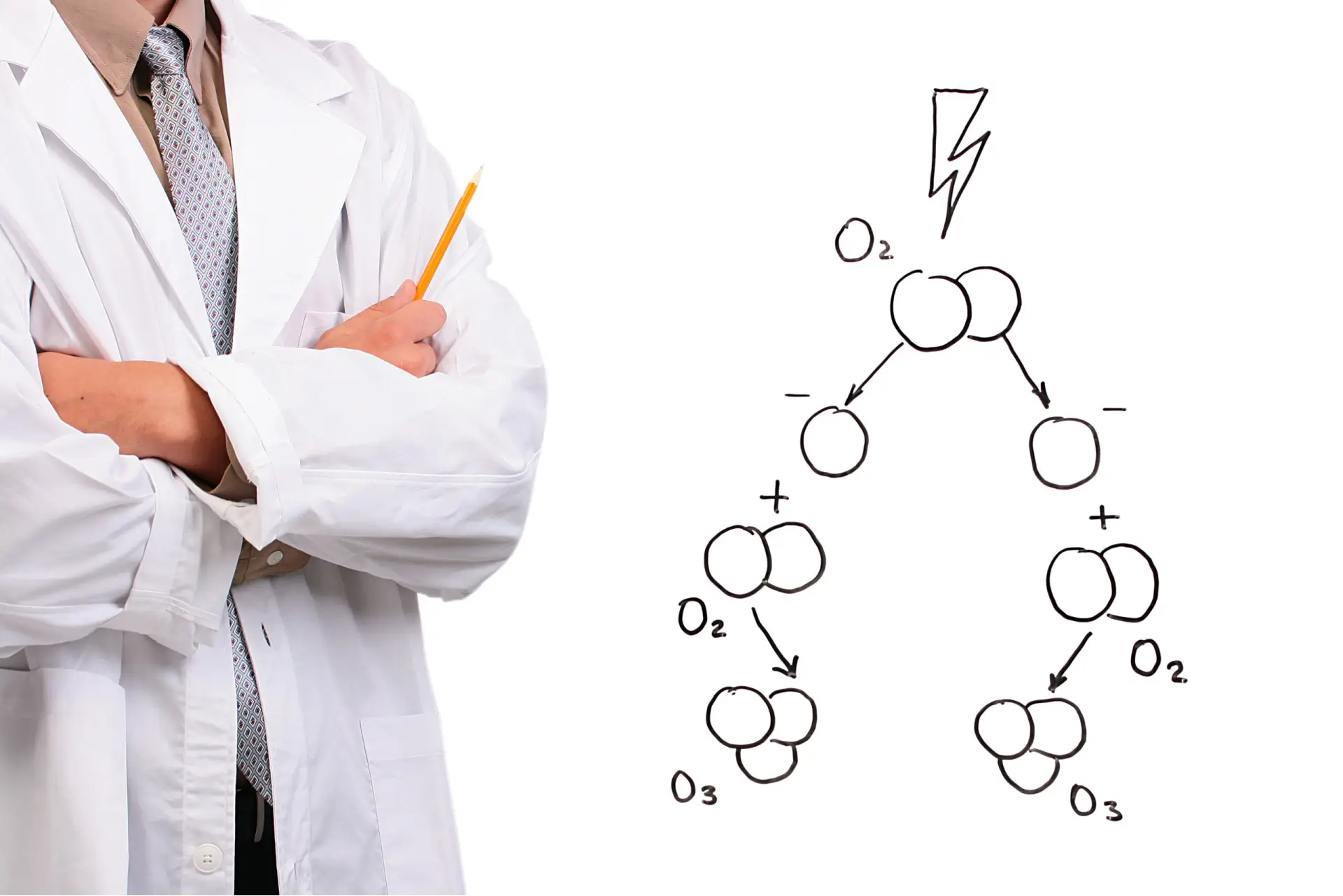
Explore the unique chemistry of ozone (O3) and its role in water treatment. Unlike other chemicals, ozone is oxygen-based and leaves no lasting residues, making it highly effective yet environmentally friendly. Its reactive yet short-lived nature ensures effectiveness without downstream concerns.
Home » Chemistry of Ozone